External Partnership Proposals
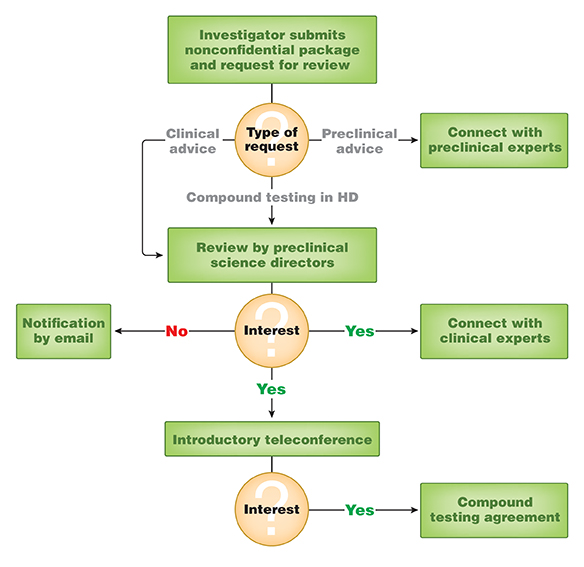
CHDI has an External Partnerships effort that is interested in learning about preclinical or clinical phase ligands from the biopharmaceutical community that may be useful for target and/or mechanistic validation studies for Huntingdon’s disease with the potential to be disease-modifying. If you have a compound of interest and would like to collaborate with CHDI to evaluate it in an HD context for proof of concept, please submit a non-confidential package for our science directors to review. CHDI’s External Partnerships team will determine whether there is interest and, if so, will work with you to develop a mutually agreeable plan. The type of a potential collaboration depends on the compound of interest and the nature of your request.
CHDI does not typically provide direct funding for preclinical evaluation of compounds. We can, under some circumstances, enter into a compound testing agreement (CTA) that allows us to validate the effect of third-party molecules with our contract research organization (CRO) colleagues. We have a wide variety of validated assays available, some of them proprietary to CHDI, including generic ADMET assays, PK/PD endpoints, biomarker assays, HD-specific cellular assays, and several phenotypic disease progression measures in HD animal models. All studies conducted at CROs under a CTA will be funded by CHDI and the resulting data is confidential. Your organization retains ownership of all intellectual property rights and data generated, and we typically do not ask for structures to be disclosed. Furthermore, you may opt to fund your own additional studies on any returned samples and/or animal tissues.
Our External Partnerships team focuses on HD-relevant projects that are at or near the proof-of-concept stage for in vivo animal testing. CHDI has internal drug discovery programs that focus on earlier stage projects, so we do not provide funding for either external drug screening activities or optimizing compounds at the hit-to-lead stage. CHDI can provide advice and point towards a variety of resources, including HD animal model testing protocols available through our network of specialized CROs.
If you are interested in a potential collaboration to evaluate your advanced preclinical or clinical phase compound, please provide the following information:
- The biological rationale for efficacy in HD,
- The molecule’s biological profile (without disclosing the structure),
- A detailed non-confidential information package about the molecule(s) that will allow us to evaluate its potential for translation to the clinic. We need to understand how advanced the molecule(s) is/are and the rationale for efficacy in HD.
Please send your full contact information and non-confidential information package to . After review and evaluation, we may set up an initial exploratory call between your team and our External Partnerships team. Please allow a few weeks for the internal review process.
Frequently Asked Questions
I have a clinical candidate for HD, how can I get involved with CHDI?
For all clinical questions, please email our clinical group at: . CHDI’s clinical group will liaise internally with the preclinical group to evaluate the scientific rationale and strength of the program before dedicating any significant time or resources to providing clinical advice.
I‘d like feedback on my HD research, can CHDI put me in touch with an expert?
If you are looking for preclinical advice, please send your specific questions and non-confidential information package to and we will connect you to a CHDI science director with the relevant expertise.
I have a novel technology that I think will advance HD therapeutic development. How can I collaborate with CHDI?
Submit a detailed non-confidential information package to and we will direct your request to the appropriate contact at CHDI.
Can CHDI help place a value on an asset?
CHDI does not provide valuations, investment guidance, or venture philanthropy.
 CHDI
CHDI